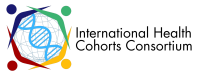IHCC Spotlight: The PERSIAN Cohort Study
The Prospective Epidemiological Research Studies in IrAN (PERSIAN), is the largest, multi-center cohort study in Iran, launched in 2014. The main goal of the PERSIAN Cohort Study is to assess the burden of major non-communicable diseases (NCDs) in Iran and identify the risk factors associated with them in order to tailor effective preventative measures and implement health-related policies (1).
The cohort includes men and women 35-70 years of age who reside in designated areas of Iran. At first, ten distinct locations were chosen as the PERSIAN Cohort centers, encompassing major ethnic populations and geographical areas of Iran, with different environmental, cultural and lifestyle exposures. But soon after the launch of the study, eight other centers were added expanding the cohort to 18 locations across Iran. The cohorts are carried out by the local Universities of Medical Sciences, under the guidance and supervision of a Central Scientific Steering Committee (CSSC) at Tehran University of Medical Sciences (Figure 1).

The PERSIAN Cohort Study methodology, sampling and data collection protocols (2) were composed by the CSSC and all measurement tools were also centrally purchased and distributed to the cohort centers to ensure consistency in data collection. In addition, one educational team trained all personnel at the 18 cohort centers in order to maintain the data as consistent as possible and limit biases when the data is pooled.
Population census was used to first identify all individuals 35-70 years of age in the designated cohort areas, then, trained personnel went door-to-door to confirm the presence of the individuals identified and then invited them to participate in the study, distributing education pamphlets. If individuals agreed to enroll, they were given an appointment date to visit the cohort center.
While the cohort centers started enrollment at various time points from 2014-2018, enrollment is currently closed at all locations with over 163,000 individuals being included in the PERSIAN Cohort. Upon enrolling, written informed consent was obtained from all participants, and biological samples (blood, urine, hair and nail) were obtained and stored in the PERSIAN Cohort Biobank after a small portion of the samples was used for routing laboratory tests. Information regarding various exposures (Table 1) was then obtained through interviewer-administered questionnaires and selective body measurements (anthropometric, pulse rate and blood pressure), were performed as well.

One year after enrollment, the follow-up phase for each individual began and individuals or their family members were contacted by phone in order to investigate the occurrence of major NCDs or potentially death in the previous year. This process is continued yearly until now. Any outcome identified during the follow-up phone calls is further investigated by collection of medical records or autopsy forms to confirm the incidence of disease or cause of death.
Initially, a repeated measurement phase was planned to be carried out five years post-enrollment, to re-collect data on various exposures in 30% of the initial cohort population. But this phase collided with the COVID-19 pandemic when all in-person cohort activities had become limited. This phase was finally carried out after COVID-19 vaccinations were performed across the country and have recently finished data collection. As part of this phase, information regarding participants’ experience with COVID, use of personal protection as well as vaccination was also collected.
The PERSIAN Cohort currently continues to collect follow-up data on the study outcomes. More information about the cohort, as well as the data collected, can be obtained at www.persiancohort.com.
REFERENCES:
- Eghtesad S, Mohammadi Z, Shayanrad A, Faramarzi E, Joukar F, Hamzeh B, et al. The PERSIAN Cohort: providing the evidence needed for healthcare reform. Archives of Iranian Medicine (2017) 20(11):691–695. PMID: 29480734.
- Hossein Poustchi, Sareh Eghtesad, Farin Kamangar, Arash Etemadi et al. Prospective Epidemiological Research Studies in Iran (the PERSIAN Cohort Study): Rationale, Objectives, and Design. American Journal of Epidemiology (2018) 187(4): 647–655. https://doi.org/10.1093/aje/kwx314.
ACKNOWLEDGMENTS:
The PERSIAN Cohort was funded by the Iranian Ministry of Health and Medical Education and local universities carrying out the study. The CSSC would like to thank all participants for engaging in this study and providing valuable information for research purposes. Without them and their patience during the data collection process, this study would not have been possible. We would also like to thank all principal investigators from the participating universities of medical sciences who used their expert research teams to best conduct this study. We hope that data collected in the PERSIAN Cohort can help shed a better light on the health of humans worldwide.
Figure 1.
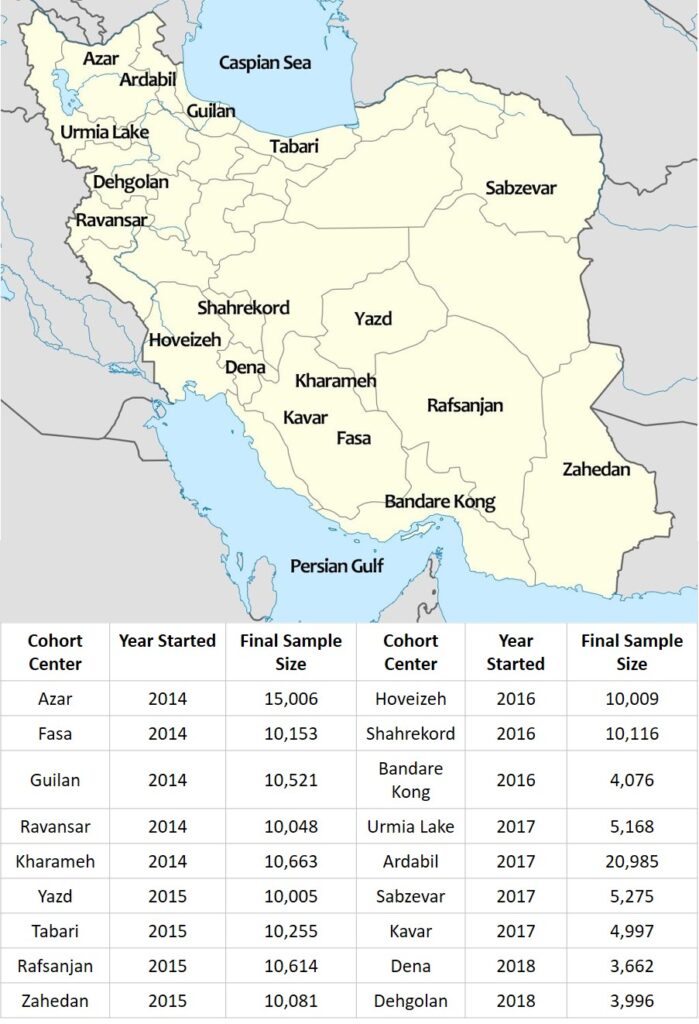
Table 1. Topic Categories in the PERSIAN Cohort questionnaires—copied from DOI: 10.1093/aje/kwx314.